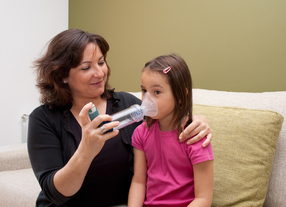
This change to legislation follows on from an overwhelmingly positive response to a UK wide consultation undertaken by MHRA. The inhalers can be administered in an emergency situation by trained staff and only to pupils who are known to have asthma.
On average, one in 11 children has asthma which means that there are approximately two children with asthma in every classroom in the UK.
The RPS have produced a reference guide to help pharmacists who may be receiving requests from schools following the changes. The reference guide is available to members of the RPS and can be accessed by clicking the link below.
RPS Supplying salbutamol inhalers to schools quick reference guide.
The guide provides information on; who can provide a signed order for inhalers and what information needs to be on it, what records a pharmacy should keep and where to go for further guidance.
The DH have produced guidance for schools. You can read this by clicking HERE
Details of the consultation which lead to the change and a summary of the change can be read HERE


 RSS Feed
RSS Feed
